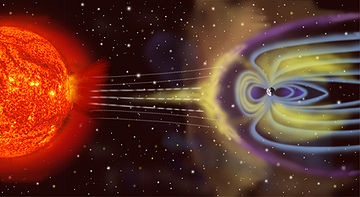
Magnetism is a property of materials that respond at an atomic or subatomic level to an applied magnetic field. Ferromagnetism is the strongest and most familiar type of magnetism. It is responsible for the behavior of permanent magnets, which produce their own persistent magnetic fields, as well as the materials that are attracted to them.
Magnetism, at its root, arises from two sources:
- Electric currents or more generally, moving electric charges create magnetic fields (see Maxwell's Equations).
- Many particles have nonzero "intrinsic" or "spin" magnetic moments. Just as each particle, by its nature, has a certain mass and charge, each has a certain magnetic moment, possibly zero.
In magnetic materials, sources of magnetization are the electrons' orbital angular motion around the nucleus, and the electrons' intrinsic magnetic moment (see electron magnetic dipole moment). The other sources of magnetism are the nuclear magnetic moments of the nuclei in the material which are typically thousands of times smaller than the electrons' magnetic moments, so they are negligible in the context of the magnetization of materials. Nuclear magnetic moments are important in other contexts, particularly in nuclear magnetic resonance (NMR) and magnetic resonance imaging (MRI).
Ordinarily, the enormous number of electrons in a material are arranged such that their magnetic moments (both orbital and intrinsic) cancel out. This is due, to some extent, to electrons combining into pairs with opposite intrinsic magnetic moments as a result of the Pauli exclusion principle (see electron configuration), or combining into filled subshells with zero net orbital motion. In both cases, the electron arrangement is so as to exactly cancel the magnetic moments from each electron. Moreover, even when the electron configuration is such that there are unpaired electrons and/or non-filled subshells, it is often the case that the various electrons in the solid will contribute magnetic moments that point in different, random directions, so that the material will not be magnetic.
However, sometimes either spontaneously, or owing to an applied external magnetic field — each of the electron magnetic moments will be, on average, lined up. Then the material can produce a net total magnetic field, which can potentially be quite strong.
The magnetic behavior of a material depends on its structure, particularly its electron configuration, for the reasons mentioned above, and also on the temperature. At high temperatures, random thermal motion makes it more difficult for the electrons to maintain alignment.
Diamagnetism:
Diamagnetism appears in all materials, and is the tendency of a material to oppose an applied magnetic field, and therefore, to be repelled by a magnetic field. Diamagnetic behavior is observed only in a purely diamagnetic material. In a diamagnetic material, there are no unpaired electrons, so the intrinsic electron magnetic moments cannot produce any bulk effect.
Paramagnetism:
In a paramagnetic material there are unpaired electrons, i.e. atomic or molecular orbitals with exactly one electron in them. While paired electrons are required by the Pauli exclusion principle to have their intrinsic (spin) magnetic moments pointing in opposite directions, causing their magnetic fields to cancel out, an unpaired electron is free to align its magnetic moment in any direction. When an external magnetic field is applied, these magnetic moments will tend to align themselves in the same direction as the applied field, thus reinforcing it.
Ferromagnetism:
A ferromagnet, like a paramagnetic substance, has unpaired electrons. However, in addition to the electrons' intrinsic magnetic moment's tendency to be parallel to an applied field, there is also in these materials a tendency for these magnetic moments to orient parallel to each other to maintain a lowered-energy state. Thus, even when the applied field is removed, the electrons in the material maintain a parallel orientation.
Every ferromagnetic substance has its own individual temperature, called the Curie temperature, or Curie point, above which it loses its ferromagnetic properties. This is because the thermal tendency to disorder overwhelms the energy-lowering due to ferromagnetic order.
Some well-known ferromagnetic materials that exhibit easily detectable magnetic properties (to form magnets) are nickel, iron, cobalt, gadolinium and their alloys.
Antiferromagnetism:
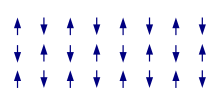 In an antiferromagnet, unlike a ferromagnet, there is a tendency for the intrinsic magnetic moments of neighboring valence electrons to point in opposite directions. When all atoms are arranged in a substance so that each neighbor is 'anti-aligned', the substance is antiferromagnetic. Antiferromagnets have a zero net magnetic moment, meaning no field is produced by them. Antiferromagnets are less common compared to the other types of behaviors, and are mostly observed at low temperatures. In varying temperatures, antiferromagnets can be seen to exhibit diamagnetic and ferrimagnetic properties.
In an antiferromagnet, unlike a ferromagnet, there is a tendency for the intrinsic magnetic moments of neighboring valence electrons to point in opposite directions. When all atoms are arranged in a substance so that each neighbor is 'anti-aligned', the substance is antiferromagnetic. Antiferromagnets have a zero net magnetic moment, meaning no field is produced by them. Antiferromagnets are less common compared to the other types of behaviors, and are mostly observed at low temperatures. In varying temperatures, antiferromagnets can be seen to exhibit diamagnetic and ferrimagnetic properties.
In some materials, neighboring electrons want to point in opposite directions, but there is no geometrical arrangement in which each pair of neighbors is anti-aligned. This is called a spin glass, and is an example of geometrical frustration.
Ferrimagnetism:
Like ferromagnetism, ferrimagnets retain their magnetization in the absence of a field. However, like antiferromagnets, neighboring pairs of electron spins like to point in opposite directions. These two properties are not contradictory, because in the optimal geometrical arrangement, there is more magnetic moment from the sublattice of electrons that point in one direction, than from the sublattice that points in the opposite direction.
The first discovered magnetic substance, magnetite, was originally believed to be a ferromagnet. It was later disproved.
Electromagnet:
An electromagnet is a type of magnet whose magnetism is produced by the flow of electric current. The magnetic field disappears when the current ceases.

 The earliest phases of the Big Bang are subject to much speculation. In the most common models the Universe was filled homogeneously and isotropically with an incredibly high energy density and huge temperatures and pressures and was very rapidly expanding and cooling. Approximately 10−37 seconds into the expansion, a phase transition caused a cosmic inflation, during which the Universe grew exponentially. After inflation stopped, the Universe consisted of a quark–gluon plasma, as well as all other elementary particles.Temperatures were so high that the random motions of particles were at relativistic speeds, and particle–antiparticle pairs of all kinds were being continuously created and destroyed in collisions. At some point an unknown reaction called baryogenesis violated the conservation of baryon number, leading to a very small excess of quarks and leptons over antiquarks and anti-leptons—of the order of one part in 30 million. This resulted in the predominance of matter over antimatter in the present Universe.
The earliest phases of the Big Bang are subject to much speculation. In the most common models the Universe was filled homogeneously and isotropically with an incredibly high energy density and huge temperatures and pressures and was very rapidly expanding and cooling. Approximately 10−37 seconds into the expansion, a phase transition caused a cosmic inflation, during which the Universe grew exponentially. After inflation stopped, the Universe consisted of a quark–gluon plasma, as well as all other elementary particles.Temperatures were so high that the random motions of particles were at relativistic speeds, and particle–antiparticle pairs of all kinds were being continuously created and destroyed in collisions. At some point an unknown reaction called baryogenesis violated the conservation of baryon number, leading to a very small excess of quarks and leptons over antiquarks and anti-leptons—of the order of one part in 30 million. This resulted in the predominance of matter over antimatter in the present Universe.













 is the internal energy of formation of the reactant molecules that can be calculated from the bond energies of the various chemical bonds of the molecules under consideration and
is the internal energy of formation of the reactant molecules that can be calculated from the bond energies of the various chemical bonds of the molecules under consideration and  is the internal energy of formation of the product molecules. The
internal energy change of a process is equal to the heat change if it is
measured under conditions of constant volume, as in a closed rigid
container such as a bomb calorimeter.
However, under conditions of constant pressure, as in reactions in
vessels open to the atmosphere, the measured heat change is not always
equal to the internal energy change, because pressure-volume work also
releases or absorbs energy. (The heat change at constant pressure is
called the enthalpy change; in this case the enthalpy of formation).
is the internal energy of formation of the product molecules. The
internal energy change of a process is equal to the heat change if it is
measured under conditions of constant volume, as in a closed rigid
container such as a bomb calorimeter.
However, under conditions of constant pressure, as in reactions in
vessels open to the atmosphere, the measured heat change is not always
equal to the internal energy change, because pressure-volume work also
releases or absorbs energy. (The heat change at constant pressure is
called the enthalpy change; in this case the enthalpy of formation).