The kinetic energy of an object is the energy which it possesses due to its motion. It is defined as the work needed to accelerate a body of a given mass from rest to its stated velocity. Having gained this energy during its acceleration, the body maintains this kinetic energy unless its speed changes. The same amount of work is done by the body in decelerating from its current speed to a state of rest.
In classical mechanics, the kinetic energy of a non-rotating object of mass m traveling at a speed v is ½ mv². In relativistic mechanics, this is only a good approximation when v is much less than the speed of light.
 Since the kinetic energy increases with the square of the speed, an object doubling its speed has four times as much kinetic energy. For example, a car traveling twice as fast as another requires four times as much distance to stop, assuming a constant braking force.
Since the kinetic energy increases with the square of the speed, an object doubling its speed has four times as much kinetic energy. For example, a car traveling twice as fast as another requires four times as much distance to stop, assuming a constant braking force.
In classical mechanics, the kinetic energy of a non-rotating object of mass m traveling at a speed v is ½ mv². In relativistic mechanics, this is only a good approximation when v is much less than the speed of light.
Kinetic energy of rigid bodies
In classical mechanics, the kinetic energy of a point object (an object so small that its mass can be assumed to exist at one point), or a non-rotating rigid body, is given by the equation
where m is the mass and v is the speed (or the velocity) of the body. In SI units (used for most modern scientific work), mass is measured in kilograms, speed in metres per second, and the resulting kinetic energy is in joules.
For example, one would calculate the kinetic energy of an 80 kg mass (about 180 lbs) traveling at 18 metres per second (about 40 mph, or 65 km/h) as
- Ek = (1/2) · 80 · 182 J = 12.96 kJ
 Since the kinetic energy increases with the square of the speed, an object doubling its speed has four times as much kinetic energy. For example, a car traveling twice as fast as another requires four times as much distance to stop, assuming a constant braking force.
Since the kinetic energy increases with the square of the speed, an object doubling its speed has four times as much kinetic energy. For example, a car traveling twice as fast as another requires four times as much distance to stop, assuming a constant braking force.
The kinetic energy of an object is related to its momentum by the equation:
where:
 is momentum
is momentum is mass of the body
is mass of the body
For the translational kinetic energy, that is the kinetic energy associated with rectilinear motion, of a rigid body with constant mass  , whose center of mass is moving in a straight line with speed
, whose center of mass is moving in a straight line with speed 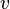 , as seen above is equal to
, as seen above is equal to
 , whose center of mass is moving in a straight line with speed
, whose center of mass is moving in a straight line with speed 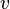 , as seen above is equal to
, as seen above is equal to
where:
 is the mass of the body
is the mass of the body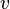 is the speed of the center of mass of the body.
is the speed of the center of mass of the body.
The kinetic energy of any entity depends on the reference frame in which it is measured. However the total energy of an isolated system, i.e. one which energy can neither enter nor leave, does not change in whatever reference frame it is measured. Thus, the chemical energy converted to kinetic energy by a rocket engine is divided differently between the rocket ship and its exhaust stream depending upon the chosen reference frame. This is called the Oberth effect. But the total energy of the system, including kinetic energy, fuel chemical energy, heat, etc., is conserved over time, regardless of the choice of reference frame. Different observers moving with different reference frames disagree on the value of this conserved energy.
The kinetic energy of such systems depends on the choice of reference frame: the reference frame that gives the minimum value of that energy is the center of momentum frame, i.e. the reference frame in which the total momentum of the system is zero. This minimum kinetic energy contributes to the invariant mass of the system as a whole.
From the work-energy theorem, we find that the total energy of a system remain constant neglecting work done against friction. Therefore we can say the change in K.E = change in P.E(U)










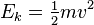
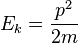
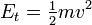









.jpg)


