Radioactivity is the emission of radiations from the nucleus of a radioactive atom. It occurs due to the unstable nucleus of the atom. The nucleus of an atom becomes unstable when the ratio of neutrons to protons is greater than 1.5.
Types of radioactivity:
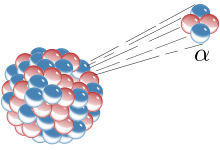 |
| Alpha Decay |
- Alpha Decay:
In an Alpha Decay, the radioactive element emits an alpha particle 'α' or simply a 4
2He2+. This results in the decrease of
atomic number(Z) as well as mass number(A) of the atom.
The atomic number decrease by 2 and the mass by 2. - Beta Decay:
β−: In a β− Decay, the nucleus emits an electron and an anti
neutrino.This results in increase of atomic
number by 1. This happens because the neutron
in the nucleus converts itself into a proton.
β+: In a β+ Decay, energy is used to convert a proton into a neutron, while emitting a positron* and a neutrino. - Neutron Emission: It is a type of radioactive decay of atoms containing excess neutrons, in which a neutron is simply ejected from the nucleus.
The most common radiations from a radioactive element are Alpha particles, Beta particles and Gamma rays(γ). The radioactivity usually starts with an alpha decay then followed by a beta decay and and finally and emission of gamma rays. The excess energy stored in a nucleus when the release of an alpha particle or beta particle is released as gamma radiations.
Properties of Alpha particles:
- They are positively charged helium 2+ ions.
- The are affected by electric fields.
- They are heavy.
- They penetrate through a body the least.
- They ionize the surroundings the most.
- Their velocity is 10^4 m/s.
Properties of Beta Particles:
- They are negatively charged particles and are electrons.
- They are also affected by electric fields.
- They are very light.
- They penetrate through a body more than alpha particles but less than Gamma rays
- They ionize the surrounding very less when compared with alpha particles.
- Their velocity is 10^6 m/s.
Properties of Gamma Rays:
- They are electromagnetic radiations.
- They are not affected by electric fields.
- They do not have any mass.
- They penetrate through the body the most. They can pass through 30cm thick graphite.
- They do not ionize the surroundings.
- Since they are electromagnetic radiations, their velocity is equal to that of light.








0 comments:
Post a Comment